INVESTOR RELATIONS CENTER
Everest Medicines

News Detail
Press Release News vom 02.06.2024
|
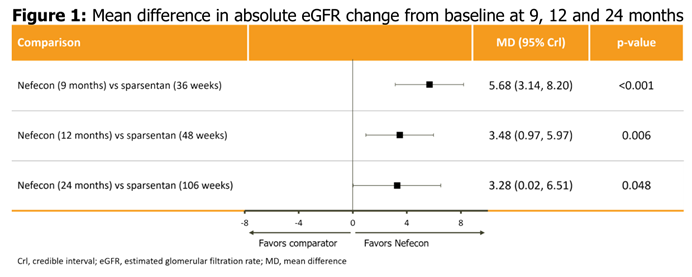
Everest Medicines' Partner Calliditas Announces an Additional Efficacy Analysis of NEFECON® at the 61st European Renal Association Congress Everest Medicines (HKEX 1952.HK, "Everest" , or the "Company”)’s licensing partner Calliditas Therapeutics AB (Nasdaq: CALT, Nasdaq Stockholm: CALTX) (“Calliditas”), has announced an additional efficacy analysis of NEFECON® in IgA nephropathy (IgAN) at the 61st European Renal Association Congress (ERA 2024) . The presented efficacy analysis of NEFECON® and sparsentan showed that treatment with NEFECON® 16 mg/day for 9 months was associated with estimated glomerular filtration rate (eGFR) benefit compared with continuous treatment with sparsentan 400 mg/day over 2 years [1]. “The positive results presented at the ERA 2024 strongly demonstrate that NEFECON® can bring stronger benefits to IgAN patients in protecting kidney function. With approximately 5 million IgAN patients in China and over 100,000 newly diagnosed patients annually, there is a significant unmet clinical demand,” said Rogers Yongqing Luo, Chief Executive Officer of Everest Medicines. “Most Chinese patients are at risk of progression to end-stage-renal-disease within their lifespan, as patients show more aggressive disease progression. Therefore, proactive treatment is needed to intervene and control such risk, delaying the need for dialysis or kidney transplantation. As the world's first-in-disease therapy for IgAN, NEFECON® has been officially commercialized in mainland China. In the future, we will continue to improve the accessibility of NEFECON® and bring this innovative therapy to more patients to meet urgent clinical needs.” The analysis is a matching-adjusted indirect comparison (MAIC) of eGFR in patients with IgAN treated with NEFECON® or sparsentan. As a measure of renal function, eGFR is accepted as a surrogate endpoint for clinical trials evaluating kidney function deterioration. An anchored MAIC was performed to estimate the relative effect of NEFECON® and sparsentan on the absolute eGFR change from baseline at 9, 12 and 24 months, with common comparators of optimized renin–angiotensin system inhibition for NefIgArd and irbesartan for PROTECT. Patient-level data from NefIgArd were used to select a population matched to PROTECT. Absolute change in eGFR was analyzed using a mixed model for repeated measures (MMRM) method, including baseline, 3-, 6-, 9-, 12-, 18-and 24-month data, baseline eGFR, baseline eGFR-by-time interaction, treatment, and treatment-by-time interaction. The MAIC weights were incorporated into the MMRM. A Bayesian fixed-effects network meta-analysis was performed on the relative effect from PROTECT and the weighted relative effect from NefIgArd, measured by the estimated absolute change from baseline in eGFR. Results from the anchored MAIC showed statistically and clinically significant favorable effects of NEFECON® versus sparsentan on eGFR for all time points analyzed (Figure 1). After accounting for differences in the patient populations from the NefIgArd and PROTECT trials, the anchored MAIC showed that treatment with NEFECON® 16 mg/day for 9 months was associated with greater eGFR benefit compared with continuous treatment with sparsentan 400 mg/day over 2 years, with significant differences observed as early as 9 months after treatment initiation and sustained up to 2-years of follow-up. It should be emphasized that, in the NefIgArd trial, patients discontinued NEFECON® after 9 months of treatment, with subsequent management consisted solely of best supportive care. This explains the observed convergence in eGFR outcomes between NEFECON® and sparsentan at 12 and 24 months.
As the world's first-ever fully FDA-approved treatment for IgAN, NEFECON® has been approved in mainland China, Macau, Hong Kong and Singapore. With the commercial launch of NEFECON® in mainland China, a Patient Assistance Program has been initiated to provide partial medication assistance to adult patients in mainland China. Reference 1. Reich H,et al. #2459-Matching-adjusted indirect comparison of eGFR in patients with immunoglobulin A nephropathy treated with Nefecon (TRF budesonide) or sparsentan. Presented at ERA 2024. About NEFECON® NEFECON® is a patented oral, delayed release formulation of budesonide, a corticosteroid with potent glucocorticoid activity and weak mineralocorticoid activity that undergoes substantial first pass metabolism. The formulation is designed as a delayed release capsule that is enteric coated so that it remains intact until it releases budesonide to the distal ileum. Each capsule contains coated beads of budesonide that target mucosal B-cells present in the ileum where the disease originates, as per the predominant pathogenesis models. In June 2019, Everest Medicines entered into an exclusive, royalty-bearing license agreement with Calliditas, which gives Everest Medicines exclusive rights to develop and commercialize NEFECON® in Mainland China, Hong Kong, Macau, Taiwan and Singapore. The agreement was extended in March 2022 to include South Korea as part of Everest Medicine's territories. About Everest Medicines Everest Medicines is a biopharmaceutical company focused on discovering, developing, manufacturing and commercializing transformative pharmaceutical products and vaccines that address critical unmet medical needs for patients in Asian markets. The management team of Everest Medicines has deep expertise and an extensive track record from both leading global pharmaceutical companies and local Chinese pharmaceutical companies in high-quality discovery, clinical development, regulatory affairs, CMC, business development and operations. Everest Medicines has built a portfolio of potentially global first-in-class or best-in-class molecules in the company’s core therapeutic areas of renal diseases, infectious diseases and autoimmune disorders. For more information, please visit its website at www.everestmedicines.com. Forward-Looking Statements: This news release may make statements that constitute forward-looking statements, including descriptions regarding the intent, belief or current expectations of the Company or its officers with respect to the business operations and financial condition of the Company, which can be identified by terminology such as “will,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “confident” and similar statements. Such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, or other factors, some of which are beyond the control of the Company and are unforeseeable. Therefore, the actual results may differ from those in the forward-looking statements as a result of various factors and assumptions, such as future changes and developments in our business, competitive environment, political, economic, legal and social conditions. The Company or any of its affiliates, directors, officers, advisors or representatives has no obligation and does not undertake to revise forward-looking statements to reflect new information, future events or circumstances after the date of this news release, except as required by law. |